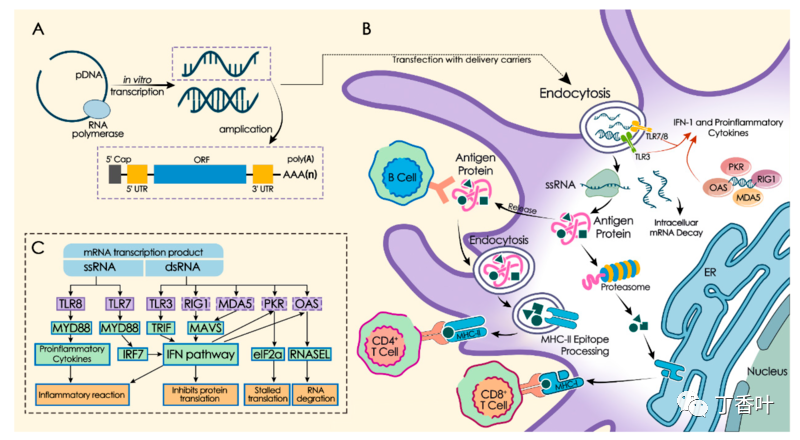
核糖核酸 (RNA) 疫苗或信使 RNA (mRNA) 疫苗是一种疫苗,它使用称为信使 RNA (mRNA) 的天然分子的副本来产生免疫反应。该疫苗将合成 RNA 分子转染到免疫细胞中。一旦进入免疫细胞,疫苗的 RNA 就会发挥 mRNA 的作用,导致细胞构建通常由病原体(如病毒)或癌细胞产生的外源蛋白质。这些蛋白质分子刺激适应性免疫反应,教会身体如何识别和破坏相应的病原体或癌细胞。mRNA 的传递是通过将分子共同配制到脂质纳米颗粒中来实现的,脂质纳米颗粒保护 RNA 链并帮助它们吸收到细胞中。
反应原性,即疫苗能够产生常见的、预期的不良反应的特性,与传统的非 RNA 疫苗相似。对自身免疫反应敏感的人可能对 RNA 疫苗有不良反应。RNA 疫苗相对于传统蛋白质疫苗的优势在于优越的设计[如何?] 和生产速度、较低的生产成本以及诱导细胞和体液免疫。辉瑞-BioNTech COVID-19 疫苗在分发前需要超低温储存,但其他 mRNA 疫苗不需要[为什么?],例如 Moderna、CureVac 和 Walvax 的 COVID-19 疫苗。
在 RNA 疗法中,mRNA 疫苗作为 COVID-19 疫苗引起了相当大的兴趣。到 2020 年 12 月,有两种 COVID-19 新型 mRNA 疫苗已完成所需的最终人体试验后八周时间并正在等待紧急使用授权 (EUA):Moderna COVID-19 疫苗 (mRNA-1273) 和辉瑞-BioNTech COVID-19 疫苗(BNT162b2)。2020 年 12 月 2 日,英国药品和保健品监管局 (MHRA) 成为首个批准 mRNA 疫苗的药品监管机构,授权辉瑞-BioNTech COVID-19 疫苗 (Comirnaty) 广泛使用。2020 年 12 月 11 日,美国食品和药物管理局 (FDA) 发布了辉瑞-BioNTech COVID-19 疫苗的 EUA,美国疾病控制与预防中心 (CDC) 于 12 月 12 日建议将其用于 16 岁及以上的人群2020. 2020 年 12 月 19 日,在 FDA 批准 EUA 后,CDC 建议在成人中使用 Moderna COVID-19 疫苗。
在疫苗中使用 RNA 一直是通过社交媒体传播的大量错误信息的基础,错误地声称使用 RNA 会改变一个人的 DNA,这本身在生物学上是不可能发生的。
历史
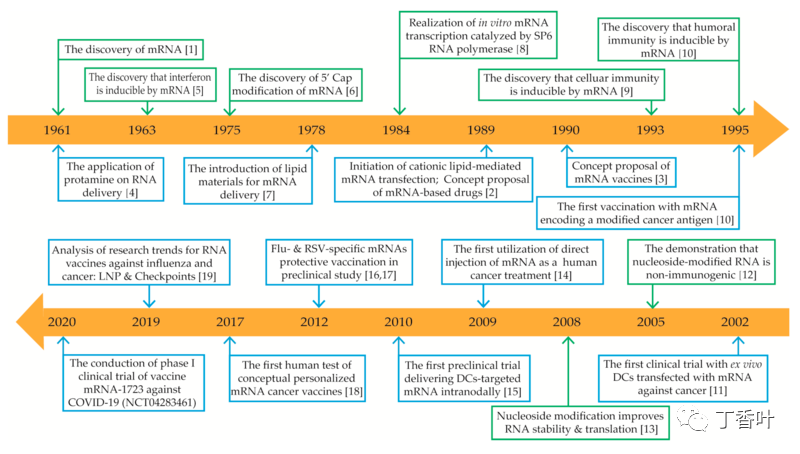
1989 年,加利福尼亚生物技术初创公司 Vical 的研究人员首次实现了 mRNA 可用于治疗目的的想法。当年与索尔克研究所合作的研究人员发表了一篇文章,表明纳米粒子可以将 mRNA 转染到细胞中。1990 年,威斯康星大学的 Jon Wolff 与 Vical 的研究人员合作,报告了将“裸”(或未受保护的)mRNA 注射到小鼠肌肉中的阳性结果。这些研究是体外转录 (IVT) mRNA 可以传递遗传信息以在活细胞组织内产生蛋白质的第一个证据。
1990 年,Vical 和威斯康星大学在动物体内证明了体外转录的 mRNA 活性,此后不久就提出了将 mRNA 用于免疫目的的提议。1993 年,Martinon 证明脂质体包裹的 RNA 可以在体内刺激 T 细胞,1994 年,Zhou 和 Berglund 发表了第一个证据,证明 RNA 可以用作疫苗来引发针对病原体的体液和细胞免疫反应。

匈牙利生物化学家 Katalin Karikó 在 1990 年代试图解决将 mRNA 引入细胞的一些主要技术障碍。Karikó 与美国免疫学家 Drew Weissman 合作,到 2005 年,他们发表了一篇联合论文,通过使用修饰的核苷在不启动身体防御系统的情况下将 mRNA 引入细胞内,解决了一个关键技术障碍。Karikó 在专注于为什么在实验中用作对照的转移 RNA 不会引起与信使 RNA 相同的免疫反应后,得出了她的关键见解。哈佛干细胞生物学家 Derrick Rossi(当时在斯坦福大学)阅读了 Karikó 和 Weissman 的论文并认识到他们的工作是“开创性的”,并于 2010 年与 Robert Langer 一起创立了以 mRNA 为重点的生物技术 Moderna,后者也看到了其在疫苗开发方面的潜力。Moderna 和 BioNTech 都获得了 Karikó 和 Weissman 工作的许可。
2010 年,美国政府机构 DARPA 启动了一项名为 ADEPT 的生物技术研究计划,作为其为美国军方开发新兴技术的使命的一部分。2011 年,DARPA 认识到核酸技术在抵御流行病方面的潜力,并开始通过 ADEPT 对该领域进行投资。DARPA 的拨款被视为信任投票,这反过来又鼓励其他政府机构和私人投资者也投资于 mRNA 技术。2013 年,DARPA 向 Moderna 授予了 2500 万美元的赠款。
直到 2020 年,这些 mRNA 生物技术公司在检测 mRNA 药物治疗心血管、代谢和肾脏疾病方面的结果都很差;选定的癌症靶点;以及 Crigler-Najjar 综合征等罕见疾病,大多数人发现 mRNA 传递方法的副作用太严重。人类使用的 mRNA 疫苗已经开发出来并针对狂犬病、寨卡病毒、巨细胞病毒和流感等疾病进行了测试,尽管这些 mRNA 疫苗尚未获得许可。许多大型制药公司放弃了这项技术,而一些生物技术公司则重新专注于利润较低的疫苗领域,在这些领域中剂量较低且副作用减少。
在 COVID-19 大流行开始时,没有任何 mRNA 药物或疫苗被批准用于人类。2020 年 12 月,Moderna 和辉瑞-BioNTech 均获得了其基于 mRNA 的 COVID-19疫苗的紧急使用授权,该疫苗由 Operation Warp Speed 资助(直接在 Moderna 的情况下,间接在辉瑞-BioNTech 的情况下)。2020 年 12 月 2 日,也就是在为期 8 周的最后试验后 7 天,英国药品和保健品监管局 (MHRA) 成为历史上第一个批准 mRNA 疫苗的全球药品监管机构,授予辉瑞-BioNTech 的 BNT162b2 COVID 紧急授权 -19疫苗广泛使用。MHRA 首席执行官琼·雷恩 (June Raine) 表示,“在批准它时没有走捷径”,而且“收益大于风险”。2020 年 12 月 11 日,FDA 授予辉瑞-BioNTech COVID-19 疫苗紧急使用授权。
机制
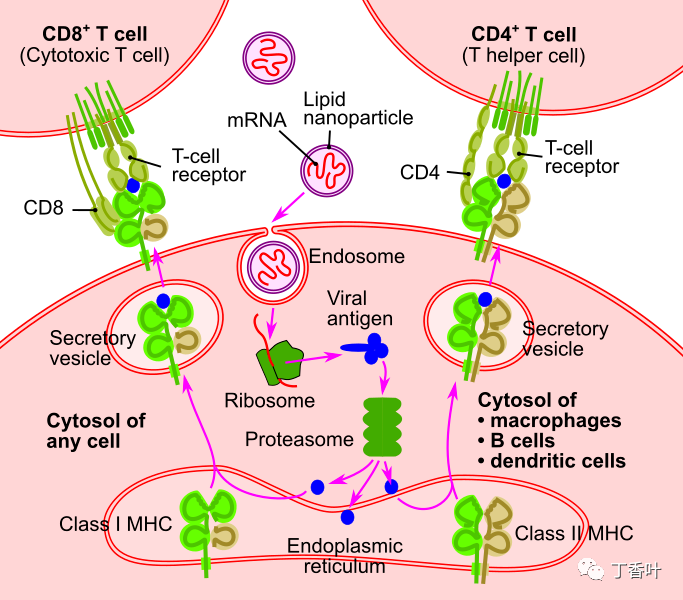
疫苗的目标是刺激适应性免疫系统产生精确针对特定病原体的抗体。抗体所针对的病原体上的标记称为抗原
mRNA 疫苗的运作方式与传统疫苗截然不同。传统疫苗通过向肌肉注射抗原、减毒病毒(减弱或无害的病毒)或重组抗原编码病毒载体(带有抗原的载体病毒)来刺激抗体反应。这些含有抗原的成分在体外制备和生长。
相比之下,mRNA 疫苗将病毒 RNA 序列的短寿命合成片段引入接种的个体。这些 mRNA 片段被树突状细胞(一种免疫系统细胞)通过吞噬作用吸收。树突细胞使用它们自己的内部机制(核糖体)来读取 mRNA 并在破坏 mRNA 之前产生 mRNA 编码的病毒抗原。尽管非免疫细胞可能会吸收疫苗 mRNA、制造尖峰并在其表面显示尖峰,但树突状细胞更容易吸收 mRNA 小球。
一旦宿主细胞产生病毒抗原,就会遵循正常的适应性免疫系统过程。抗原被蛋白酶体分解,然后 I 类和 II 类 MHC 分子附着在抗原上并将其运输到细胞膜,“激活”树突细胞。[未通过验证] 一旦树突细胞被激活,它们就会迁移到淋巴结,其中抗原呈递给 T 细胞和 B 细胞。这最终会导致产生专门针对抗原的抗体,从而产生免疫力。
使用 mRNA 使宿主细胞产生抗原的好处是,疫苗制造者比抗原蛋白或减毒病毒更容易产生 mRNA。另一个好处是设计和生产的速度。Moderna 在 2 天内为 COVID-19 设计了 mRNA-1273 疫苗。RNA 疫苗的另一个优点是,由于抗原是在细胞内产生的,它们可以刺激细胞免疫以及体液免疫。
mRNA 疫苗不会影响或重新编程细胞内的 DNA。合成的 mRNA 片段是病毒 RNA 特定部分的副本,它携带着构建病毒抗原的指令(在主要的冠状病毒 mRNA 疫苗的情况下是蛋白质刺突),与人类 DNA 无关。这种误解随着 COVID-19 mRNA 疫苗的出现而广为流传,是一个被揭穿的阴谋论。
产生外源蛋白质后,mRNA 应在细胞中降解。然而,由于 mRNA 疫苗的制造商对具体配方(包括脂质纳米颗粒药物递送涂层的确切成分)保密,因此第三方尚未研究细节和时间。
递送
疫苗递送的方法可以根据向细胞的 RNA 转移发生在生物体内(体内)还是体外(离体)来大致分类。
离体
树突状细胞是一种免疫细胞,其表面显示抗原,导致与 T 细胞相互作用以启动免疫反应。可以从患者身上收集树突细胞并用所需的 mRNA 进行编程。然后,它们可以重新给药回患者以产生免疫反应。
体内
由于发现在直接给药后引入体外转录的 mRNA 导致体内表达,体内方法变得越来越有吸引力。与离体方法相比,它们具有一些优势,特别是通过避免从患者身上收获和适应树突细胞的成本,以及通过模拟常规感染。要使 RNA 疫苗接种成为一种有效的程序,这些方法仍然需要克服一些障碍。需要绕过阻止未知核酸材料渗透和促进 RNase 降解的进化机制以启动翻译。此外,RNA 太重,无法通过扩散在细胞内自行移动,因此很容易被宿主细胞发现和消除。
裸体mRNA注射
裸注射意味着疫苗的递送被简单地保存在缓冲液中。这种 mRNA 摄取模式自 2000 年代以来就已为人所知。第一个全球临床研究(德国图宾根)使用皮内注射裸体 mRNA 进行疫苗接种。
1990 年代发现了使用 RNA 作为疫苗工具的自扩增 mRNA 的形式。mRNA 疫苗的两大类是非扩增型(常规,病毒递送)和分子自扩增 mRNA(非病毒递送)。当 mRNA 以非病毒方式传递时,它会进入细胞的细胞质,并可以扩增和表达抗原蛋白。
还发现不同的注射途径,例如进入皮肤、血液或肌肉,导致不同水平的 mRNA 摄取,使得给药途径的选择成为递送的关键方面。一项研究表明,在比较不同的途径时,淋巴结注射会导致最大的 T 细胞反应。
自放大 mRNA 的机制和因此的评估可能不同,因为自放大 mRNA 是一个更大的分子,因此根本不同。
复合矢量
阳离子聚合物可以与 mRNA 混合以产生称为复合物的保护涂层。这些保护重组 mRNA 免受核糖核酸酶的影响并帮助其渗透到细胞中。鱼精蛋白是一种天然阳离子肽,已被用于封装 mRNA 以进行疫苗接种。
脂质纳米粒子载体
FDA 首次批准使用脂质纳米颗粒作为药物递送系统是在 2018 年,当时该机构批准了第一种 siRNA 药物 Onpattro。将 mRNA 分子封装在脂质纳米颗粒中是生产可行的 mRNA 疫苗的关键突破,解决了将 mRNA 分子输送到宿主细胞中的许多关键技术障碍。使用脂质向细胞传递 siRNA 的研究成为类似研究使用脂质传递 mRNA 的基础。然而,必须发明新的脂质来封装比 siRNA 链长得多的 mRNA 链。
病毒载体
副作用和风险
一般
贮存
DNA疫苗
疫苗犹豫
COVID-19 mRNA 疫苗的功效
自扩增RNA
Park KS, Sun X, Aikins ME, Moon JJ (December 2020). “Non-viral COVID-19 vaccine delivery systems”. Advanced Drug Delivery Reviews. 169: 137–51. doi:10.1016/j.addr.2020.12.008. PMC 7744276. PMID 33340620.
Kowalski PS, Rudra A, Miao L, Anderson DG (April 2019). “Delivering the Messenger: Advances in Technologies for Therapeutic mRNA Delivery”. Mol Ther. 27 (4): 710–28. doi:10.1016/j.ymthe.2019.02.012. PMC 6453548. PMID 30846391.
Verbeke R, Lentacker I, De Smedt SC, Dewitte H (October 2019). “Three decades of messenger RNA vaccine development”. Nano Today. 28: 100766. doi:10.1016/j.nantod.2019.100766.
Pardi N, Hogan MJ, Porter FW, Weissman D (April 2018). “mRNA vaccines – a new era in vaccinology”. Nature Reviews. Drug Discovery. 17 (4): 261–79. doi:10.1038/nrd.2017.243. PMC 5906799. PMID 29326426.
PHG Foundation (2019). “RNA vaccines: an introduction”. University of Cambridge. Retrieved 18 November 2020.
Kramps T, Elders K (2017). “Introduction to RNA Vaccines”. RNA Vaccines: Methods and Protocols. Methods in Molecular Biology. 1499. pp. 1–11. doi:10.1007/978-1-4939-6481-9_1. ISBN 978-1-4939-6479-6. PMID 27987140.
“UK authorises Pfizer/BioNTech COVID-19 vaccine” (Press release). Department of Health and Social Care. 2 December 2020.
Boseley S, Halliday J (2 December 2020). “UK approves Pfizer/BioNTech Covid vaccine for rollout next week”. The Guardian. Retrieved 2 December 2020.
“Conditions of Authorisation for Pfizer/BioNTech COVID-19 Vaccine” (Decision). Medicines & Healthcare Products Regulatory Agency. 8 December 2020.
“FDA Takes Key Action in Fight Against COVID-19 By Issuing Emergency Use Authorization for First COVID-19 Vaccine”. U.S. Food and Drug Administration (FDA) (Press release). 11 December 2020. Retrieved 6 February 2021.
Oliver SE, Gargano JW, Marin M, Wallace M, Curran KG, Chamberland M, et al. (December 2020). “The Advisory Committee on Immunization Practices’ Interim Recommendation for Use of Pfizer-BioNTech COVID-19 Vaccine – United States, December 2020” (PDF). MMWR Morb Mortal Wkly Rep. 69 (50): 1922–24. doi:10.15585/mmwr.mm6950e2. PMC 7745957. PMID 33332292.
“FDA Takes Additional Action in Fight Against COVID-19 By Issuing Emergency Use Authorization for Second COVID-19 Vaccine”. U.S. Food and Drug Administration (FDA) (Press release). 18 December 2020.
Oliver SE, Gargano JW, Marin M, Wallace M, Curran KG, Chamberland M, et al. (January 2021). “The Advisory Committee on Immunization Practices’ Interim Recommendation for Use of Moderna COVID-19 Vaccine – United States, December 2020” (PDF). MMWR Morb Mortal Wkly Rep. 69 (5152): 1653–56. doi:10.15585/mmwr.mm695152e1. PMID 33382675. S2CID 229945697.
Carmichael F, Goodman J (2 December 2020). “Vaccine rumours debunked: Microchips, ‘altered DNA’ and more” (Reality Check). BBC.
Malone, R. W.; Felgner, P. L.; Verma, I. M. (1 August 1989). “Cationic liposome-mediated RNA transfection”. Proceedings of the National Academy of Sciences. 86 (16): 6077–6081.
Xu, Shuqin; Yang, Kunpeng; Li, Rose; Zhang, Lu (January 2020). “mRNA Vaccine Era—Mechanisms, Drug Platform and Clinical Prospection”. International Journal of Molecular Sciences. 21 (18): 6582. doi:10.3390/ijms21186582. PMC 7554980. Initiation of cationic lipid-mediated mrna transfection; Concept proposal of mRNA-based drugs.
Wolff, Jon A.; Malone, Robert W.; Williams, Phillip; Chong, Wang; Acsadi, Gyula; Jani, Agnes; Felgner, Philip L. (23 March 1990). “Direct Gene Transfer into Mouse Muscle in Vivo”. Science. 247 (4949): 1465–1468. doi:10.1126/science.1690918. ISSN 0036-8075. PMID 1690918.
Pardi, N., Hogan, M., Porter, F. et al. (2018). “— a new era in vaccinology”, Nature Rev. Drug Discov., 17, pp. 261–79
Sahin U, Karikó K, Türeci Ö (October 2014). “mRNA-based therapeutics – developing a new class of drugs”. Nature Reviews. Drug Discovery. 13 (10): 759–80. doi:10.1038/nrd4278. PMID 25233993. S2CID 27454546.
Weissman D (February 2015). “mRNA transcript therapy”. Expert Review of Vaccines. 14 (2): 265–81. doi:10.1586/14760584.2015.973859. PMID 25359562. S2CID 39511619.
Patent: WO1990011092; Inventors: Philip L. Felgner, Jon Asher Wolff, Gary H. Rhodes, Robert Wallace Malone, Dennis A. Carson; Assignees: Vical Inc., Wisconsin Alumni Researh Foundation; Title:”Expression of Exogenous Polynucleotide Sequences in a Vertebrate”; Priority date: 1989-03-21; Issue date: 1990-10-04.
Pascolo S (August 2004). “Messenger RNA-based vaccines”. Expert Opinion on Biological Therapy. 4 (8): 1285–94. doi:10.1517/14712598.4.8.1285. PMID 15268662. S2CID 19350848.
Kallen KJ, Theß A (January 2014). “A development that may evolve into a revolution in medicine: mRNA as the basis for novel, nucleotide-based vaccines and drugs”. Therapeutic Advances in Vaccines. 2 (1): 10–31. doi:10.1177/2051013613508729. PMC 3991152. PMID 24757523.
Garade D (10 November 2020). “The story of mRNA: How a once-dismissed idea became a leading technology in the Covid vaccine race”. Stat. Retrieved 16 November 2020.
Kolata, Gina (8 April 2021). “Kati Kariko Helped Shield the World From the Coronavirus”. The New York Times. ISSN 0362-4331. Retrieved 8 April 2021.
Sonne, Paul (30 July 2020). “How a secretive Pentagon agency seeded the ground for a rapid coronavirus cure”. The Washington Post.
Usdin, Steve (19 March 2020). “DARPA’s gambles might have created the best hopes for stopping COVID-19”. BioCentury. Retrieved 19 June 2021.
“DARPA Awards Moderna Therapeutics A Grant For Up To $25 Million To Develop Messenger RNA Therapeutics”. 2 October 2013. Retrieved 31 May 2021.
Garde D (10 January 2017). “Lavishly funded Moderna hits safety problems in bold bid to revolutionize medicine”. Stat. Archived from the original on 16 November 2020. Retrieved 19 May 2020. struggling to get mRNA into cells without triggering nasty side effects
Garade D (13 September 2016). “Ego, ambition, and turmoil: Inside one of biotech’s most secretive startups”. Stat. Archived from the original on 16 November 2020. Retrieved 18 May 2020. because it’s exceedingly hard to get RNA into cells without triggering nasty side effects
“COVID-19 and Your Health”. Centers for Disease Control and Prevention. 11 February 2020.
Kuznia R, Polglase K, Mezzofiore G (1 May 2020). “In quest for vaccine, US makes ‘big bet’ on company with unproven technology”. CNN Investigates. Archived from the original on 16 November 2020. Retrieved 1 May 2020.
Roberts M (2 December 2020). “Covid Pfizer vaccine approved for use next week in UK”. BBC News. Retrieved 2 December 2020.
“UK regulator says it did not cut any corners to approve Pfizer vaccine”. Reuters. 2 December 2020. Retrieved 2 December 2020.
“The benefits of the Pfizer/BioNTech vaccine “far outweigh any risk”, says Dr June Raine from UK regulator MHRA”. BBC News Twittter. 2 December 2020. Retrieved 2 December 2020.
Guarascio F (2 December 2020). “EU criticises ‘hasty’ UK approval of COVID-19 vaccine”. Reuters. Retrieved 2 December 2020.
Commissioner, Office of the (18 December 2020). “Pfizer-BioNTech COVID-19 Vaccine”. FDA.
Batty CJ, Heise MT, Bachelder EM, Ainslie KM (December 2020). “Vaccine formulations in clinical development for the prevention of severe acute respiratory syndrome coronavirus 2 infection”. Advanced Drug Delivery Reviews. 169: 168–89. doi:10.1016/j.addr.2020.12.006. PMC 7733686. PMID 33316346.
Hajj KA, Whitehead KA (12 September 2017). “Tools for translation: non-viral materials for therapeutic mRNA delivery”. Nature Reviews Materials. 2 (10): 17056. Bibcode:2017NatRM…217056H. doi:10.1038/natrevmats.2017.56.
Schlake T, Thess A, Fotin-Mleczek M, Kallen KJ (November 2012). “Developing mRNA-vaccine technologies”. RNA Biology. 9 (11): 1319–30. doi:10.4161/rna.22269. PMC 3597572. PMID 23064118.
Goldman, Bruce (22 December 2020). “How do the new COVID-19 vaccines work?”. Scope. Stanford Medicine. Retrieved 28 January 2021.
“Seven vital questions about the RNA Covid-19 vaccines emerging from clinical trials”. Wellcome Trust. 19 November 2020. Retrieved 26 November 2020.
Fiedler K, Lazzaro S, Lutz J, Rauch S, Heidenreich R (2016). “mRNA Cancer Vaccines”. Recent Results in Cancer Research. Fortschritte der Krebsforschung. Progres dans les Recherches Sur le Cancer. Recent Results in Cancer Research. 209: 61–85. doi:10.1007/978-3-319-42934-2_5. ISBN 978-3-319-42932-8. PMID 28101688.
Neilson S, Dunn A, Bendix A (26 November 2020). “Moderna’s groundbreaking coronavirus vaccine was designed in just 2 days”. Business Insider. Retrieved 28 November 2020.
Dolgin E (November 2020). “COVID-19 vaccines poised for launch, but impact on pandemic unclear”. Nature Biotechnology. doi:10.1038/d41587-020-00022-y. PMID 33239758. S2CID 227176634.
Carmichael F (15 November 2020). “Vaccine rumours debunked: Microchips, ‘altered DNA’ and more”. BBC News. Retrieved 17 November 2020.
Rahman G (30 November 2020). “RNA Covid-19 vaccines will not change your DNA”. Full Fact. Retrieved 1 December 2020.
Vallejo J (18 November 2020). “‘What is Covid vaccine made of?’ trends on Google as Pfizer and Moderna seek FDA approval”. The Independent. Retrieved 3 December 2020.
Benteyn D, Heirman C, Bonehill A, Thielemans K, Breckpot K (February 2015). “mRNA-based dendritic cell vaccines”. Expert Review of Vaccines. 14 (2): 161–76. doi:10.1586/14760584.2014.957684. PMID 25196947. S2CID 38292712.
Wolff JA, Malone RW, Williams P, Chong W, Acsadi G, Jani A, Felgner PL (March 1990). “Direct gene transfer into mouse muscle in vivo”. Science. 247 (4949 Pt 1): 1465–68. Bibcode:1990Sci…247.1465W. doi:10.1126/science.1690918. PMID 1690918.
“Vaccine components”. Immunisation Advisory Centre. 22 September 2016. Retrieved 20 December 2020.
Probst J, Weide B, Scheel B, Pichler BJ, Hoerr I, Rammensee HG, Pascolo S (August 2007). “Spontaneous cellular uptake of exogenous messenger RNA in vivo is nucleic acid-specific, saturable and ion dependent”. Gene Therapy. 14 (15): 1175–80. doi:10.1038/sj.gt.3302964. PMID 17476302.
Lorenz C, Fotin-Mleczek M, Roth G, Becker C, Dam TC, Verdurmen WP, et al. (July 2011). “Protein expression from exogenous mRNA: uptake by receptor-mediated endocytosis and trafficking via the lysosomal pathway”. RNA Biology. 8 (4): 627–36. doi:10.4161/rna.8.4.15394. PMID 21654214.
Zhou X, Berglund P, Rhodes G, Parker SE, Jondal M, Liljeström P (December 1994). “Self-replicating Semliki Forest virus RNA as recombinant vaccine”. Vaccine. 12 (16): 1510–14. doi:10.1016/0264-410x(94)90074-4. PMID 7879415.
Berglund P, Smerdou C, Fleeton MN, Tubulekas I, Liljeström P (June 1998). “Enhancing immune responses using suicidal DNA vaccines”. Nature Biotechnology. 16 (6): 562–65. doi:10.1038/nbt0698-562. PMID 9624688. S2CID 38532700.
Deering RP, Kommareddy S, Ulmer JB, Brito LA, Geall AJ (June 2014). “Nucleic acid vaccines: prospects for non-viral delivery of mRNA vaccines”. Expert Opin Drug Deliv. 11 (6): 885–99. doi:10.1517/17425247.2014.901308. PMID 24665982. S2CID 33489182.
Geall AJ, Verma A, Otten GR, Shaw CA, Hekele A, Banerjee K, Cu Y, Beard CW, Brito LA, Krucker T, O’Hagan DT, Singh M, Mason PW, Valiante NM, Dormitzer PR, Barnett SW, Rappuoli R, Ulmer JB, Mandl CW (September 2012). “Nonviral delivery of self-amplifying RNA vaccines”. Proc Natl Acad Sci U S A. 109 (36): 14604–09. Bibcode:2012PNAS..10914604G. doi:10.1073/pnas.1209367109. PMC 3437863. PMID 22908294.
Kreiter S, Selmi A, Diken M, Koslowski M, Britten CM, Huber C, et al. (November 2010). “Intranodal vaccination with naked antigen-encoding RNA elicits potent prophylactic and therapeutic antitumoral immunity”. Cancer Research. 70 (22): 9031–40. doi:10.1158/0008-5472.can-10-0699. PMID 21045153.
[non-primary source needed]Weide B, Pascolo S, Scheel B, Derhovanessian E, Pflugfelder A, Eigentler TK, Pawelec G, Hoerr I, Rammensee HG, Garbe C (June 2009). “Direct injection of protamine-protected mRNA: results of a phase 1/2 vaccination trial in metastatic melanoma patients”. J Immunother. 32 (5): 498–507. doi:10.1097/CJI.0b013e3181a00068. PMID 19609242. S2CID 3278811.
Cooney E (1 December 2020). “How nanotechnology helps mRNA Covid-19 vaccines work”. Stat. Retrieved 3 December 2020.
Reichmuth AM, Oberli MA, Jaklenec A, Langer R, Blankschtein D (May 2016). “mRNA vaccine delivery using lipid nanoparticles”. Therapeutic Delivery. 7 (5): 319–34. doi:10.4155/tde-2016-0006. PMC 5439223. PMID 27075952.
Cross, Ryan (6 March 2021). “Without these lipid shells, there would be no mRNA vaccines for COVID-19”. Chemical & Engineering News. American Chemical Society. Retrieved 6 March 2021.
Paunovska K, Sago CD, Monaco CM, Hudson WH, Castro MG, Rudoltz TG, et al. (March 2018). “A Direct Comparison of in Vitro and in Vivo Nucleic Acid Delivery Mediated by Hundreds of Nanoparticles Reveals a Weak Correlation”. Nano Letters. 18 (3): 2148–57. Bibcode:2018NanoL..18.2148P. doi:10.1021/acs.nanolett.8b00432. PMC 6054134. PMID 29489381.
Lowe, Derek (3 February 2021). “Opinion: A straightforward explanation why more COVID-19 vaccines can’t be produced with help from ‘dozens’ of companies”. MarketWatch. Retrieved 5 February 2021.
King, Anthony (23 March 2021). “Why manufacturing Covid vaccines at scale is hard”. Chemistry World. Royal Society of Chemistry. Retrieved 26 March 2021.
Sealy, Amanda (2 April 2021). “Manufacturing moonshot: How Pfizer makes its millions of Covid-19 vaccine doses”. CNN.
Weise, Elizabeth; Weintraub, Karen (7 February 2021). “Race to the Vaccine: A COVID-19 vaccine life cycle: from DNA to doses”. USA Today. Gannett. Retrieved 24 February 2021.
Hopkins, Jared S.; Eastwood, Joel; Moriarty, Dylan (3 March 2021). “mRNA Covid-19 Vaccines Are Fast to Make, but Hard to Scale”. The Wall Street Journal.
Rowland, Christopher (18 February 2021). “Why grandparents can’t find vaccines: Scarcity of niche biotech ingredients”. The Washington Post.
Lundstrom K (March 2019). “RNA Viruses as Tools in Gene Therapy and Vaccine Development”. Genes. 10 (3): 189. doi:10.3390/genes10030189. PMC 6471356. PMID 30832256.
Huang TT, Parab S, Burnett R, Diago O, Ostertag D, Hofman FM, et al. (February 2015). “Intravenous administration of retroviral replicating vector, Toca 511, demonstrates therapeutic efficacy in orthotopic immune-competent mouse glioma model”. Human Gene Therapy. 26 (2): 82–93. doi:10.1089/hum.2014.100. PMC 4326030. PMID 25419577.
Schultz-Cherry S, Dybing JK, Davis NL, Williamson C, Suarez DL, Johnston R, Perdue ML (December 2000). “Influenza virus (A/HK/156/97) hemagglutinin expressed by an alphavirus replicon system protects chickens against lethal infection with Hong Kong-origin H5N1 viruses”. Virology. 278 (1): 55–59. doi:10.1006/viro.2000.0635. PMID 11112481.
Geisbert TW, Feldmann H (November 2011). “Recombinant vesicular stomatitis virus-based vaccines against Ebola and Marburg virus infections”. The Journal of Infectious Diseases. 204 (Suppl 3): S1075–81. doi:10.1093/infdis/jir349. PMC 3218670. PMID 21987744.
Wadman M (November 2020). “Public needs to prep for vaccine side effects”. Science. 370 (6520): 1022. doi:10.1126/science.370.6520.1022. PMID 33243869.
Thomas K (22 October 2020). “Experts Tell F.D.A. It Should Gather More Safety Data on Covid-19 Vaccines”. New York Times. Retrieved 21 November 2020.
Kuchler H (30 September 2020). “Pfizer boss warns on risk of fast-tracking vaccines”. Financial Times. Retrieved 21 November 2020.
“Pfizer-BioNTech COVID-19 Vaccine Vaccination Storage & Dry Ice Safety Handling”. Pfizer. Retrieved 17 December 2020.
Simmons-Duffin S. “Why Does Pfizer’s COVID-19 Vaccine Need To Be Kept Colder Than Antarctica?”. NPR.org. Retrieved 18 November 2020.
“Fact Sheet for Healthcare Providers Administering Vaccine” (PDF). ModernaTX, Inc.
“Moderna Announces Longer Shelf Life for its COVID-19 Vaccine Candidate at Refrigerated Temperatures”. NPR.org.
Rabson, Mia (27 February 2021). “From science to syringe: COVID-19 vaccines are miracles of science and supply chains”. CTV News. Bell Media.
Karikó K, Buckstein M, Ni H, Weissman D (August 2005). “Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA”. Immunity. 23 (2): 165–75. doi:10.1016/j.immuni.2005.06.008. PMID 16111635.
Karikó K, Muramatsu H, Ludwig J, Weissman D (November 2011). “Generating the optimal mRNA for therapy: HPLC purification eliminates immune activation and improves translation of nucleoside-modified, protein-encoding mRNA”. Nucleic Acids Research. 39 (21): e142. doi:10.1093/nar/gkr695. PMC 3241667. PMID 21890902.
Pardi N, Weissman D (17 December 2016). “Nucleoside Modified mRNA Vaccines for Infectious Diseases”. RNA Vaccines. Methods in Molecular Biology. 1499. Springer New York. pp. 109–21. doi:10.1007/978-1-4939-6481-9_6. ISBN 978-1-4939-6479-6. PMID 27987145.
Schlake T, Thess A, Fotin-Mleczek M, Kallen KJ (November 2012). “Developing mRNA-vaccine technologies”. RNA Biology. 9 (11): 1319–30. doi:10.4161/rna.22269. PMC 3597572. PMID 23064118.
Vogel AB, Lambert L, Kinnear E, Busse D, Erbar S, Reuter KC, et al. (February 2018). “Self-Amplifying RNA Vaccines Give Equivalent Protection against Influenza to mRNA Vaccines but at Much Lower Doses”. Molecular Therapy. 26 (2): 446–55. doi:10.1016/j.ymthe.2017.11.017. PMC 5835025. PMID 29275847.
Skalka AM (2014). “Retroviral DNA Transposition: Themes and Variations”. Microbiology Spectrum. 2 (5): 1101–23. doi:10.1128/microbiolspec.MDNA3-0005-2014. ISBN 9781555819200. PMC 4383315. PMID 25844274.
Nirenberg, Edward (24 November 2020). “No, Really, mRNA Vaccines Are Not Going To Affect Your DNA”. Vaccines, Immunology, COVID-19. deplatformdisease. Retrieved 28 January 2021.
Rowland C (21 November 2020). “Doctors and nurses want more data before championing vaccines to end the pandemic”. Washington Post. Retrieved 22 November 2020.
Kwon D (25 November 2020). “The Promise of mRNA Vaccines”. The Scientist. Retrieved 27 November 2020.
Jaffe-Hoffman M (17 November 2020). “Could mRNA COVID-19 vaccines be dangerous in the long-term?”. The Jerusalem Post. Retrieved 17 November 2020.
Bloom, Kristie; van den Berg, Fiona; Arbuthnot, Patrick (22 October 2020). “Self-amplifying RNA vaccines for infectious diseases”. Gene Therapy. 28 (3–4): 117–29. doi:10.1038/s41434-020-00204-y. PMC 7580817. PMID 33093657.
“saRNA Biology | About Self-Amplifying RNA Genome & How It Works”. Chimeron Bio | Transforming RNA Therapy.
Lowe, Derek (1 March 2021). “A Malaria Vaccine Candidate”. Science Translational Medicine. Retrieved 7 May 2021.
所有分享及看法仅限专业人士交流及参考
参考及图片等来源于网络,版权归原作者所有
微信公众号“丁香叶”ID:dxyeyx