Abstract
Angiogenesis is involved not only in pathological conditions including cancer biology and non-neoplastic diseases, but also many biological processes including reproduction, development and repair. During angiogenesis, endothelial cells (ECs) undergo activation after binding of angiogenic factors to their receptors, release of proteases to dissolve the basement membrane, migration towards an angiogenic signal, proliferation, and an increase in cell number for new blood vessel formation. Finally, reorganization of ECs forms the three-dimensional vasculature. HUVEC tube-formation assay is one of the simple, but well-established in vitro angiogenesis assays based on the ability of ECs to form three-dimensional capillary-like tubular structures, when cultured on a gel of growth factor-reduced basement membrane extracts. During the assay, ECs differentiate, directionally migrate to align, branch, and form the tubular polygonal networks of blood vessels.
Keywords: HUVEC (人脐静脉内皮细胞), Angiogenesis assay (血管生成实验), Tube formation assay (管形成实验)
Materials and Reagents
- Target cell lines can be those with and without drug treatment or expressing the gene of interest.
Note: Our example includes four cell lines that are engineered to have inducible expression of tumor suppressor gene and the corresponding vector alone controls. - Growth factor-reduced BD Matrigel (BD Biosciences, catalog number: 354230 )
- Dulbecco’s Modified Eagle Medium (DMEM) (High Glucose L-Glutamine, 500 ml) (Life Technologies, Invitrogen™, catalog number: 11965-092 )
- Fetal bovine serum (Life Technologies, Invitrogen™, catalog number: 26140-079 )
- Primary Human Umbilical Vein Endothelial Cell (HUVEC) (Life Technologies, Invitrogen™, catalog number: C-003-5C )
- Medium 200PRF (Life Technologies, Invitrogen™, catalog number: M-200PRF-500 )
- Low serum growth supplement (LSGS) (Life Technologies, Invitrogen™, catalog number: S-003-10 )
- Conditioned medium (CM)
Equipment
- Tissue culture setup
- Inverted microscope with digital camera (Nikon TMS)
- Scion Image software downloaded from the NIH website
- 96-well plates
- Centrifuge
- Cell counter
- T75 tissue culture flask
Procedure
- Preparation of conditioned medium from target cell line(s)
- To prepare conditioned medium (CM), seed target cells and grow to 30-40% confluence (depending on the growth rate of the cell lines), replace growth medium with serum-free DMEM (e.g. 10 ml for a T75 tissue culture flask; it does not matter for the presence or absence of antibiotics) for 24 h, then harvest the CM, when cells reach 60-80% confluence in a T75 tissue culture flask.
- Make 0.5 ml aliquots of the CM and store at -80 °C if it is not immediately used after collection of CM.
- Preparation of Human Umbilical Vein Endothelial Cells (HUVEC) cells
- Prepare the HUVEC Medium 200PRF supplement with LSGS according to the product insert.
- Seed HUVEC cells in T25 or T75 flask, as needed, so that it reaches 70-80% confluence by the next day.
- Serum starve the HUVEC cells for 3-6 h just prior to performing the tube formation assay in Medium 200PRF without antibiotics (e.g. 5 ml for a T25 tissue culture flask).
Note: At the end of serum starvation, go to procedure C step 1. - After coating the 96-well plate with growth factor-reduced Matrigel, trypsinize to dislodge the cells from the surface of the flask, neutralize with DMEM with serum, pellet down the cells at room temperature by centrifugation at 1,200 rpm ( 276 x g) for 3 min, resuspend in 2-3 ml serum-free DMEM, determine the concentration of HUVEC by counting the cells, and resuspend HUVEC cells at 4 x 105 per ml serum-free DMEM by pipetting up and down several times to ensure a homogeneous single-cell suspension.
Note: For the growth factor-reduced Matrigel, thaw/frozen cycle should be reduced as much as possible. - Mix thoroughly while pipetting 500 μl of the HUVEC cell suspension into a 1.5 ml tube. Spin down the cells at 4,000 rpm (1,100 rcf) for 3 min using a bench-top centrifuge. Carefully aspirate the supernatant without disturbing the cell pellet. Remove as much of the supernatant as possible. Prepare the appropriate number of HUVEC cells in 1.5 ml tubes, according to the number of target cell lines to be used.
Note: Per target cell line CM, one tube of HUVEC will be suspended, and per suspended HUVEC, 3 triplicate wells will be dispensed. So, in total, 3 wells will be used per target cell line. - Thaw a 0.5 ml aliquot of the CM of target cell line(s) collected from procedure A step 2 and supplement it with fetal bovine serum to a final concentration of 1% for resuspending the HUVEC cell pellet(s) in procedure B step 5.
Note: Each target cell line should be done in triplicate (each well requires 100 μl of the HUVEC cell suspension).
- Coating of 96-well plate with growth factor-reduced Matrigel
- Thaw an appropriate volume of growth factor-reduced Matrigel one day before use at 4 °C.
- Pre-chill the 96-well plate and the pipet tips at -20 °C for 2-3 h.
- Near the end of serum starvation of HUVEC cells and before counting HUVEC cells, aliquot 50 μl of growth factor-reduced Matrigel per well of the pre-chilled 96-well plate on ice. Swirl the plate until the gel is evenly distributed over the whole well. It is very important to avoid bubble formation. Allow it to polymerize at room temperature for 1 h on a level surface.
- Dispensing HUVEC cells in onto coated 96-well plate
- Dispense 100 μl of the HUVEC cell suspension obtained in procedure B step 6 with thorough mixing into the labeled wells of a 96-well plate. Incubate the plate at 37 °C, 5% CO2 for 4-6 h.
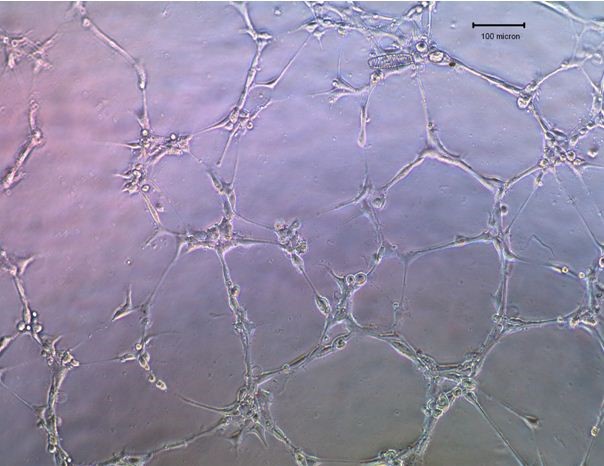
- Visualize the cells using a light microscope. Take pictures of the images of the capillary network and count the tube lengths using the Scion Image software.
Acknowledgments
The protocol was established based on modifications from previous publications: Chan et al. (2011) and Kong et al. (2007).
References
- Chan, K. C., Ko, J. M., Lung, H. L., Sedlacek, R., Zhang, Z. F., Luo, D. Z., Feng, Z. B., Chen, S., Chen, H., Chan, K. W., Tsao, S. W., Chua, D. T., Zabarovsky, E. R., Stanbridge, E. J. and Lung, M. L. (2011). Catalytic activity of Matrix metalloproteinase-19 is essential for tumor suppressor and anti-angiogenic activities in nasopharyngeal carcinoma. Int J Cancer 129(8): 1826-1837.
- Kong, D., Li, Y., Wang, Z., Banerjee, S. and Sarkar, F. H. (2007). Inhibition of angiogenesis and invasion by 3,3′-diindolylmethane is mediated by the nuclear factor-kappaB downstream target genes MMP-9 and uPA that regulated bioavailability of vascular endothelial growth factor in prostate cancer. Cancer Res 67(7): 3310-3319.
原文见:
In vitro Human Umbilical Vein Endothelial Cells (HUVEC) Tube-formation Assay —BIO-PROTOCOL
Ko, J. M. and Lung, M. L. (2012). In vitro Human Umbilical Vein Endothelial Cells (HUVEC) Tube-formation Assay. Bio-protocol 2(18): e260. DOI: 10.21769/BioProtoc.260.